

Batch Number: FRAMECPSR
Product Name: Lauric Acid
Product Number: 5060330638613
Brand: Naturallythinking
Supplier:
UK:
Naturallythinking, Unit 2 Mill Lane Trading Estate, Mill Lane, Croydon, CR0 4AA
EU: NTEU Ltd (HE 426545), Nikis 1, Anthoupoli, 2350, Nicosia, Cyprus
Contact Telephone: 020 38563588
Contact Email: support@naturallythinking.eu
2.1 Classification of the substance or mixture
CLASSIFICATION (EC 1272/2008)
2.2 Label elements According to Regulation (EC) No. 1272/2008 (CLP)
2.2.1 Label elements
GHS Product Identifier: Lauric AcidHazard pictogram(s)
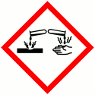

HAZARD STATEMENTS
PRECAUTION STATEMENTS
Inci: Lauric Acid
Cas: 143-07-7
Einecs:
| CAS | Hazardous Components (Chemical Name)/ REACH Registration No. | Concentration | EC No./ EC Index No | GHS Classification |
| 143-07-7 | Dodecanoic acid | 100% | 205-582-1 | Eye Damage 1: H318 |
4.1 Description of First Aid
In Case of Inhalation:
Remove to fresh air. If not breathing, give artificial respiration or give oxygen by trained personnel. Get immediate medical attention
In Case of Skin Contact:
Immediately wash skin with soap and plenty of water for at least 15 minutes. Remove contaminated clothing. Get medical attention if symptoms occur. Wash clothing before reuse.
In Case of Eye Contact:
Hold eyelids apart and flush eyes with plenty of water for at least 15 minutes. Have eyes examined and tested by medical personnel.
In Case of Ingestion:
Wash out mouth with water provided person is conscious. Never give anything by mouth to an unconscious person. Get medical attention. Do NOT induce vomiting unless directed to do so by medical personnel.
No toxicological data available
GMO Declaration
We declare that at present all our regisered and commercially available ingredients, extracts, oils, plants and skincare products are derived by traditional plant breeding methods. We do not have any ingredient, extract, oil or skincare product from genetically modified material
To the best of our knowledge, the information contained in the sheet is correct. However, we cannot accept responsibility for any consequences from its use.
Animal Testing Declaration
UK: As set out under the conditions in Schedule 34 - Amendment of Regulation (EC) No 1223/2009 and related amendments of The Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 neither the cosmetic nor its component product has been tested on animal purposes
EU:As set out in under the conditions in 1223/2009. Art. 15 Nar. Parliament and the Council of the EU 952/2013 neither the cosmetic nor its component product has been tested on animal purposes
WE DO NOT TEST ANY OF OUR PRODUCTS ON ANIMALS AND NEVER HAVE, WE PREFER TO RELY ON OUR KNOWLEDGE OF ESSENTIAL OILS, INGREDIENTS, PLANTS, FLOWERS AND ESTABLISHED TECHNICAL INFORMATION AND PROFESSIONALS TO ENSURE EFFECTIVE AND SAFE PRODUCTS.
We believe there is already a “safe” and known group of product ingredients used in the cosmetics industry which leave enough scope to be creative and innovative in product formulation without the need for anymore animal testing on new experimental ingredients.
Our suppliers each also agree to not to carry out animal testing on the products and ingredients we purchase. We have a self-imposed cut-off date of 2006 (this was the date our cosmetic formulations came into being) although in reality our suppliers normally have animal testing dates far in excess of this or have never tested at all.
We do not conduct any form of animal testing.
We do not commission any form of animal testing from a third party.
We operate a fixed cut-off date for animal testing and ingedients, which means we will not use any ingredient that has been tested or retested on animals for cosmetics purposes since January 1, 2006
If we find that a company is involved in animal testing in anyway that purchases from us, then we will cease supply immediately. It is a term and condition that by purchasing from Naturallythinking you will not involve yourself or your company in any form of Animal Testing, any violation of this will be dealt with as per any violation of our terms and condtions