

Batch Number: FRAMECPSR
Product Name: Coco Glucoside, Glyceryl Oleate
Product Number: 5060330639429
Brand: Naturallythinking
Supplier:
UK:
Naturallythinking, Unit 2 Mill Lane Trading Estate, Mill Lane, Croydon, CR0 4AA
EU: NTEU Ltd (HE 426545), Nikis 1, Anthoupoli, 2350, Nicosia, Cyprus
Contact Telephone: 020 38563588
Contact Email: support@naturallythinking.eu
2.1 Classification of the substance or mixture
CLASSIFICATION (EC 1272/2008)
2.2 Label elements According to Regulation (EC) No. 1272/2008 (CLP)
2.2.1 Label elements
GHS Product Identifier: Coco Glucoside, Glyceryl OleateHazard pictogram(s)

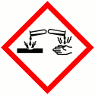
HAZARD STATEMENTS
PRECAUTION STATEMENTS
Inci: Coco Glucoside, Glyceryl Oleate
Cas: 141464-42-8/ 25496-72-4 / 111-03-5
Einecs: N/A/ 247-038-6
| Ingredient | Percentage % |
| Coco-Glucoside | 30 - 40 |
| Glyceryl Oleate | 10 - 30% |
| Aqua | < 60% |
Based on raw material used the product is EASILY BIODEGRABLE > 90% (calculated)
4.1 Description of First Aid Measures
Eye Contact: Obtain medical attention. If substance has got into the eyes, immediately wash out with plenty of water for at least 10 minutes.
Skin Contact: Remove contaminated clothing and wash affected skin with plenty of water.Wash contaminated clothing before reuse. If symptoms develop, obtain medical attention.
Inhalation: Unlikely route of exposure. Remove persons affected by vapour to fresh air. Apply artificial respiration if breathing has ceased or shows signs of failing. Administer oxygen if necessary. If symptoms develop, obtain medical attention
Ingestion: Obtain medical attention. Provided the patient is conscious, wash out mouth with water and give 200-300 ml (half a pint) of water to drink. Do not induce vomiting.
4.2 Most important symptoms and effects, both acute and delayed: Irritation, Coughing, Nausea, Vomiting
4.3 indication of the immediate medical attention and special treatment needed: Increased difficultly breathing
Form: Viscous Liquid / Yellow(ish)
Specific Gravity: 1.050 ÷ 1.100
10.1 Reactivity
Reacts with Strong oxidising agents and acids
10.2 Chemical stability
Stable under normal conditions.
10.3 Possibility of hazardous reactions
None known
10.4 Conditions to avoid
High temperatures
10.5 Incompatible materials
Strong oxidising agents. Strong acids
10.6 Hazardous Decomposition Product(s)
None known
No toxicological data available
12.1 Toxicity
(Fish) (96hrs): NOEC> 0.4mg/l (> limit of solubility)(Stearyl alcohol)
(Daphnia magna) (48hrs): EC50 > 0.01 mg/L (> limit of solubility)(Cetyl alcohol)
(Algae) (72hrs): EC50 > 0.01 mg/l (> limit of solubility)(Cetyl alcohol)
12.2 Persistence and degradability
Readily biodegradable.
12.3 Bioaccumulative potential (96 hrs)
The product has moderate potential for bioaccumulation.
12.4 Mobility in soil
This product is predicted to degrade in soil.
12.5 Results of PBT and vPvB assessment
Not classified as PBT or vPvB.
12.6 Other adverse effects
None recorded
13.1 Waste treatment methods
Material to be disposed of as hazardous waste. Normal disposal is via incineration operated by an accredited disposal contractor. Consult an accredited waste disposal contractor or the local authority for advice.
13.2 Additional Information
None
14.1 Land transport (ADR/RID)
UN number: Not classified according to the United Nations ‘Recommendations on the Transport of Dangerous Goods’.
14.2 Sea transport (IMDG)
UN number: Not classified according to the United Nations ‘Recommendations on the Transport of Dangerous Goods’.
14.3 Air transport (ICAO/IATA)
UN number: Not classified according to the United Nations ‘Recommendations on the Transport of Dangerous Goods’.
- 1907/2006/EEC, REACH
- Dir. 76/768/EEC
- Reg. 1223/2009/EEC
- California Proposition 65
- EC COSMETIC GUIDELINE / 7th MODIFICATION GUIDELINE:
There has never made any animal test for the production of cosmetic raw materials.
The product doesn’t contain any nanomaterials according to the new European Cosmetic Regulation 1223/2009/EC and any nanotechnology is used to produce it
- The product is not from animal origin. Furthermore it doesn’t contain any ingredient of animal origin, it is not produced using ingredients of animal origins and it doesn’t come into contact with animal origin ingredients at any stage of its production. It is therefore BSE free.
- Raw material of vegetal origin is GMO-free
| Criteria | Fixed Cut off Date |
|
We do not conduct any form of animal testing |
January 1, 2006 |
|
We do not commission any form of animal testing from a third party |
January 1, 2006 |
|
We operate a fixed cut-off date for animal testing and ingredients, which means we will not use any ingredient that has been tested or retested on animals for cosmetics purposes since January 1, 2006 |
January 1, 2006 |
GMO Declaration
We declare that at present all our regisered and commercially available ingredients, extracts, oils, plants and skincare products are derived by traditional plant breeding methods. We do not have any ingredient, extract, oil or skincare product from genetically modified material
To the best of our knowledge, the information contained in the sheet is correct. However, we cannot accept responsibility for any consequences from its use.
Animal Testing Declaration
UK: As set out under the conditions in Schedule 34 - Amendment of Regulation (EC) No 1223/2009 and related amendments of The Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 neither the cosmetic nor its component product has been tested on animal purposes
EU:As set out in under the conditions in 1223/2009. Art. 15 Nar. Parliament and the Council of the EU 952/2013 neither the cosmetic nor its component product has been tested on animal purposes
WE DO NOT TEST ANY OF OUR PRODUCTS ON ANIMALS AND NEVER HAVE, WE PREFER TO RELY ON OUR KNOWLEDGE OF ESSENTIAL OILS, INGREDIENTS, PLANTS, FLOWERS AND ESTABLISHED TECHNICAL INFORMATION AND PROFESSIONALS TO ENSURE EFFECTIVE AND SAFE PRODUCTS.
We believe there is already a “safe” and known group of product ingredients used in the cosmetics industry which leave enough scope to be creative and innovative in product formulation without the need for anymore animal testing on new experimental ingredients.
Our suppliers each also agree to not to carry out animal testing on the products and ingredients we purchase. We have a self-imposed cut-off date of 2006 (this was the date our cosmetic formulations came into being) although in reality our suppliers normally have animal testing dates far in excess of this or have never tested at all.
We do not conduct any form of animal testing.
We do not commission any form of animal testing from a third party.
We operate a fixed cut-off date for animal testing and ingedients, which means we will not use any ingredient that has been tested or retested on animals for cosmetics purposes since January 1, 2006
If we find that a company is involved in animal testing in anyway that purchases from us, then we will cease supply immediately. It is a term and condition that by purchasing from Naturallythinking you will not involve yourself or your company in any form of Animal Testing, any violation of this will be dealt with as per any violation of our terms and condtions